16
2024
-
09
Tsinghua University Xiao Bailong/Peking University Ouyang Kunfu Collaboration Reveals Key Phosphorylation Sites That Regulate Piezo1 Mechanical Sensitivity and Mechanotransduction Function in Vivo
Author:
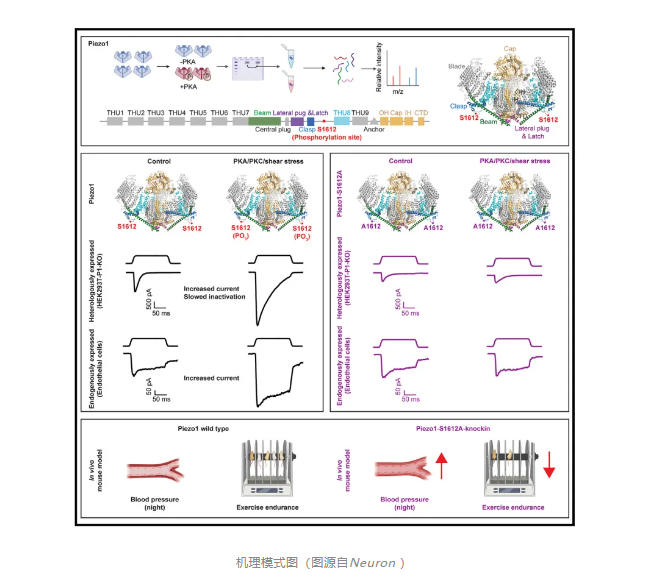
Piezo1 is a mechanically activated cation channel that can convert mechanical forces into various physiological processes.Due to its large protein size of more than 2500 amino acids and complex 38 transmembrane helix topology, how Piezo1 is post-translationally modified to regulate its mechanotransduction function in vivo remains unexplored.
On September 12, 2024, Xiao Bailong of Tsinghua University and Ouyang Kunfu of Peking University jointly communicated inNeuron(IF=14.7)Published online entitled“Phosphorylation of Piezo1 at a single residue, serine-1612, regulates its mechanosensitivity and in vivo mechanotransduction function”The research paper,The study showedPhosphorylation of Piezo1 at a single residue, serine -1612, modulates its mechanosensitivity and mechanotransduction function in vivo.
Structure-function studies of mouse Piezo1 (mPiezo1) and mPiezo2 revealed their 38-transmembrane (TM) topological folding characteristics of 2,547 and 2,822 amino acids, respectively, which trimerize to form a three-lobed, propeller-like channel and undergo a force-induced conformational transition from a highly bent state to a flattened state. The unique structural design and excellent deformability of Piezo1/2 may explain their superb mechanical sensitivity as multifunctional mechanotransduction channels in a variety of cell types and physiological processes.Despite significant progress in understanding the physiological importance and structure-function relationships of piezoelectric channels, how they can be post-translationally modified to fine-tune their channel properties to mediate their multifunctional mechanotransduction functions remains largely unexplored.
Previous
Previous
LATEST NEWS
2024-09-16
Tsinghua University Xiao Bailong/Peking University Ouyang Kunfu Collaboration Reveals Key Phosphorylation Sites That Regulate Piezo1 Mechanical Sensitivity and Mechanotransduction Function in Vivo
Piezo1 is a mechanically activated cation channel that can convert mechanical forces into various physiological processes. Due to its large protein size of more than 2500 amino acids and complex 38 transmembrane helix topology, how Piezo1 is post-translationally modified to regulate its mechanotransduction function in vivo remains unexplored.
2024-09-18
Huazhong University of Science and Technology Wang Cong Yi/Sun Fei found in obese environment really pathogenic adipose tissue macrophage subsets
Adipose tissue macrophages (ATMs) play an important role in maintaining adipose tissue homeostasis and coordinating metabolic inflammation. Given the extensive functional heterogeneity and phenotypic plasticity of ATMs, there is a need to identify truly pathogenic subpopulations of ATMs in the context of obesity.
2024-09-14
Wang Su's team at Southeast University found that microenvironmental glial cells regulate stem cell self-renewal and differentiation by delivering iron to neural stem cells through ferritin
The study of neural stem cells is of great significance for the treatment of neural development and nervous system diseases. However, the regulation mechanism of neural stem cells has not been fully elucidated, especially the regulation of neural stem cells by microenvironment is relatively less known.
2024-09-14
Subversion of the past! Tsinghua University Dai Qionghai/Guo Zengcai/Wu Jiamin Cooperation Latest Cell
A comprehensive understanding of physiopathological processes requires non-invasive live three-dimensional (3D) imaging on different spatial and temporal scales. However, huge data throughput, optical non-uniformity, surface irregularities, and phototoxicity pose huge challenges, resulting in inevitable trade-offs between volume size, resolution, speed, sample health, and system complexity.
2024-09-11
The latest research by Guo Jianping/Cheng Chao/Bo Lang of Sun Yat-sen University confirms that palmitic acid can suppress virus infection by activating innate immunity!
Innate immunity is the primary defense against viral and microbial infections. The exact effect of cellular metabolites, particularly fatty acids, on antiviral innate immunity remains largely elusive.

