05
2024
-
09
Liao Maofu Group of Southern University of Science and Technology reveals the transport and inhibition mechanism of Mycobacterium tuberculosis drug efflux pump
Author:
Tuberculosis (TB) is a global infectious disease mainly caused by Mycobacterium tuberculosis (MTB).In recent years, there have been more than 10 million new cases of tuberculosis and more than 1.6 million deaths worldwide each year, making MTB the second leading infectious agent in the world, second only to SARS-CoV-2 and surpassing HIV.drug-resistant tuberculosisThe emergence and continued increase in the global tuberculosis epidemic has become more severe.
There are two main mechanisms of MTB resistance: one is the drug target-related gene mutation leading to drug failure, and the other is the activation and overexpression of MTB drug efflux pump, which reduces the effective drug concentration in bacteria.Efflux pump A (EfpA) is the first drug efflux pump found in MTB and is required for MTB survival. In a variety of clinical drug-resistant strains, the overexpression of EfpA increased the tolerance of MTB to isoniazid, rifampicin, amikacin and other drugs by tens or even hundreds of times. A recently reported BRD-8000 series and BRD-9327 of novel anti-MTB inhibitors have been shown to specifically target EfpA.However, our understanding of the function of EfpA in MTB and its inhibitory mechanism is still limited.
On September 4, 2024,Liao Maofu Team of Southern University of Science and TechnologyInNature Communications Published online entitled“Structures of the Mycobacterium tuberculosis efflux pump EfpA reveal the mechanisms of transport and inhibition”The research paper,The studyIt is shown that EfpA may act as a lipid transporter to promote the turnover of lipids between phospholipid bilayers in MTB cells, and the site of action of the novel inhibitor on EfpA and its inhibitory mechanism are revealed.
The research team used cryo-electron microscopy to resolve the high-resolution structure of EfpA binding to endogenous lipids or inhibitors in the open-out conformation.EfpA belongs to the Major Facilitator Superfamily(MFS) superfamily of transporters, but unlike the classical 12-transmembrane helix MFS transporter,In addition to six transmembrane helices each for NTD and CTD, EfpA has a hinge domain (HD) containing two transmembrane helices connecting NTD and CTD.
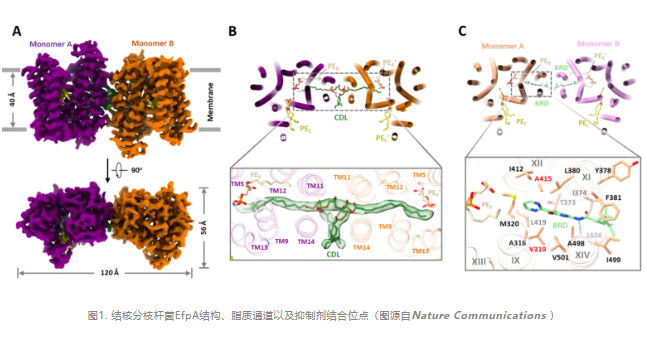
Interestingly, there is a "ladder" type channel extending from the inner lobe to the outer lobe of the plasma membrane phospholipid bilayer in EfpA, in which three lipid molecules are combined in a head-to-head and tail-to-tail manner (A, B and C positions).Molecular dynamics simulations indicate that these lipid molecules can be stably bound within the channel. In the middle region of the channel, the hydrophilic heads of the two lipid molecules together with the surrounding helical structure form a negatively charged pocket that opens outward.The size and surface charge of this pocket matches that of the hydrophilic substrates EtBr and isoniazid that are efflux of EfpA.
In the structure where EfpA binds to the inhibitor BRD-8000.3, BRD-8000.3 replaces the-site lipid molecule, occupying the inner-lobe lipid-binding site of the lipid channel in EfpA. This is consistent with the previously reported mutation sites of several BRD-8000.3 tolerant mutants. The BRD-9327 resistant mutation is located around the lipid binding site B. By AutoDock molecular docking analysis, the BRD-9327 can bind to the lipid binding site B, displacing the lipid at that site.BRD-9327 and BRD-8000.3 bind at different sites in EfpA, which explains the previously reported synergistic inhibitory effect of the two when used in combination.
Small molecules occupy the lipid binding site in EfpA, which in turn inhibits MTB, suggesting that these lipids are critically linked to the intrinsic function of EfpA in MTB.In order to further explore the intrinsic function of EfpA, the researchers used the gene structure function prediction tools COFACTOR and Dali to search the database respectively, and the two search results consistently used the lysophospholipid transport protein MFSD2A in the MFS superfamily as the best match. Despite the low sequence homology (11%),EfpA and MFSD2A exhibit a high degree of structural similarity. In addition, the two lipid binding sites B and C also correspond to each other.These findings suggest that EfpA is likely to function as a lipid transport protein, similar to MFSD2A.
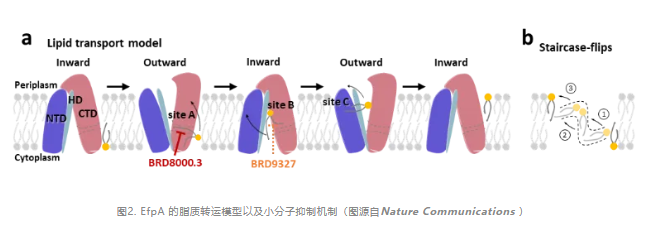
In addition, the researchers also compared the cryo-electron microscope structure of EfpA with the AlphaFold predicted structure of EfpA, revealing the conformational changes and changes in lipid channels between the two.Finally, they propose a "ladder flip" model of EfpA lipid transport, and a mechanism of action for small molecule inhibitors.
In summary, this study analyzed the structure of Mycobacterium tuberculosis multidrug efflux pump EfpA, indicating that EfpA may perform the function of lipid transport protein in MTB, and determined the binding site of the new MTB inhibitor in EfpA.These advances provide a structural basis for the research and optimization of new anti-tuberculosis drugs targeting EfpA, and advance the understanding of the mechanism of MFS superfamily lipid transport proteins.
Former Harvard University postdoctoral Dr. Wang Shuhui (now Yale University) and Professor Liao Maofu of Southern University of Science and Technology are the co-corresponding authors of this article, and Dr. Wang Shuhui is also the first author of this article. The research was strongly supported by the National Natural Science Foundation of China.
Chongqing Chongfan Technology Co., Ltd. Optical platform film thickness meter OCT optical coherence tomography LabVIEW control
LATEST NEWS
2024-09-14
Wang Su's team at Southeast University found that microenvironmental glial cells regulate stem cell self-renewal and differentiation by delivering iron to neural stem cells through ferritin
The study of neural stem cells is of great significance for the treatment of neural development and nervous system diseases. However, the regulation mechanism of neural stem cells has not been fully elucidated, especially the regulation of neural stem cells by microenvironment is relatively less known.
2024-09-14
Subversion of the past! Tsinghua University Dai Qionghai/Guo Zengcai/Wu Jiamin Cooperation Latest Cell
A comprehensive understanding of physiopathological processes requires non-invasive live three-dimensional (3D) imaging on different spatial and temporal scales. However, huge data throughput, optical non-uniformity, surface irregularities, and phototoxicity pose huge challenges, resulting in inevitable trade-offs between volume size, resolution, speed, sample health, and system complexity.
2024-09-11
The latest research by Guo Jianping/Cheng Chao/Bo Lang of Sun Yat-sen University confirms that palmitic acid can suppress virus infection by activating innate immunity!
Innate immunity is the primary defense against viral and microbial infections. The exact effect of cellular metabolites, particularly fatty acids, on antiviral innate immunity remains largely elusive.
2024-09-11
He Chuan and other teams have shown that METTL14 regulates insulin sensitivity throughout the body through a mechanism independent of UCP1!
Brown adipose tissue (BAT) regulates systemic metabolism by releasing signaling lipids. N6-methyladenosine (m6 A), the most prevalent and abundant post-transcriptional mRNA modification, has been reported to regulate BAT adipogenesis and energy expenditure.
2024-09-09
Engelie net new use! The latest research from Southern Medical University shows that SGLT2 inhibitors promote ketogenic and improve MASH by inhibiting CD8 T cell activation.
Accumulation of autoaggressive CD8 T cells contributes significantly to liver injury and inflammation during the progression of metabolic dysfunction-associated steatohepatitis (MASH). EMPA, a highly selective sodium-glucose co-transporter 2 (SGLT2) inhibitor, has potential therapeutic effects on hepatic steatosis; however, the underlying mechanism has not been fully elucidated.

